20 October 2017 | Astell Scientific Ltd
Astell''s new autoclave software for FDA 21 CFR Part 11 standard
Astell introduces autoclave controller software that meets electronic signature guidelines required for pharmaceutical and medical manufacturing industries.
Astell Scientific, the UK’s leading autoclave manufacturer, is pleased to announce the launch of a new software option that enables its full range of autoclaves to meet FDA 21 CFR Part 11 electronic signature regulations.The software is targeted towards customers operating in the pharmaceutical and medical manufacturing industries, but can bring benefits to a much wider range of applications should traceability be of key importance when specifying a sterilizer.
The basic requirement of the Electronic Records; Electronic Signature final rule (21 CFR 11) legislation is to ensure that any computerized system used for regulatory purposes produces electronic records that have integrity and reliability, and that electronic signatures are trustworthy and equivalent to handwritten signatures executed on paper records.
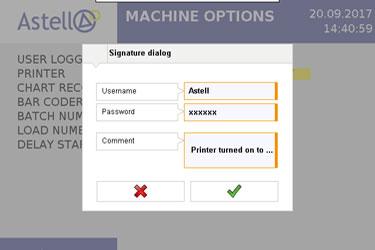
Astell’s latest software combined with its advanced touchscreen controller positions the company as one of few autoclave manufacturers able to provide a solution that complies with FDA 21 CFR Part 11. Available across both circular and square section sterilizers, the specially developed 21 CFR 11 controller software captures any change made to a cycle, option, or device parameter − which will require an electronic signature authorisation.
All device changes and signatures will be electronically logged and can be recalled to provide a clear picture of user input for any given point in time. All audit trails are saved in a file format that cannot be altered in any way. Astell spokesperson Dave Thomas commented “The introduction of FDA compliant autoclave software offers our customers the transparency and record management required by tightly regulated industries. It’s another good reason to choose Astell Scientific, and demonstrates why we remain the leaders in sterilization.” For more information on Astell’s FDA 21 CFR Part 11 software option, its applications or capabilities please visit www.astell.com/21cfr11.